Determining how Fat and the Hippo pathway regulate mouse kidney development
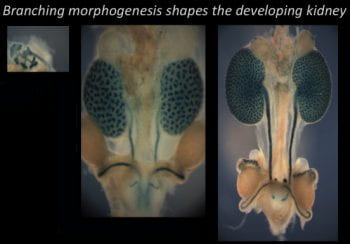 Determine how Fat cadherins regulate kidney development.
Determine how Fat cadherins regulate kidney development.
We showed that Fat cadherins control PCP in kidney development, and that loss of Fat pathway components leads to cystic disease and defects in nephron progenitor self-renewal and epithelial-mesenchymal transitions. We will use genetic analysis to dissect the contribution of Fat1-4 and Dchs1-2 binding proteins to these Fat pathway functions in vivo. We will also use high-resolution time-lapse imaging in kidney organ culture to explore the effects of loss of Fat4, and Fat4-pathway components. We recently found that loss of Fat4 leads to migration defects in the mouse brain raising defective migration as a potential cause of kidney defects. We will also examine the role of Fat4, and Fat4-binding proteins (identified in our MS analysis).
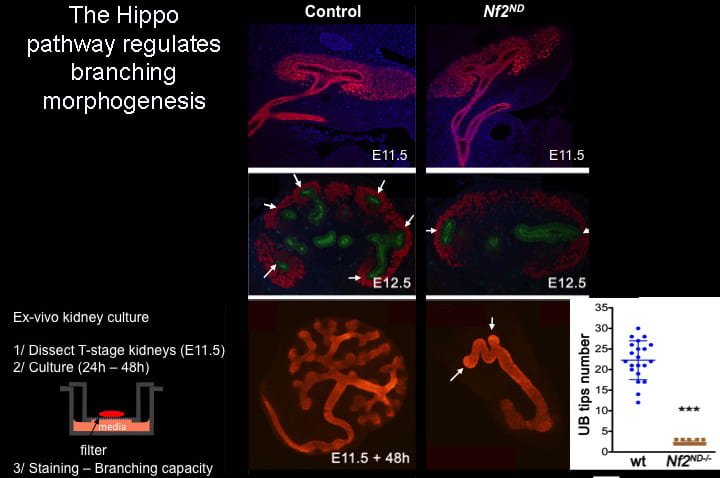
Explore the Hippo pathway in branching morphogenesis.
Using conditional knockouts in embryos, as well as organ-culture approaches, we discovered that loss of NF2 or LATS or overexpression of YAP leads to growth and elongation of kidney collecting ducts in the absence of branching (below). To investigate branching defects, we will conduct high-resolution time-lapse imaging of the induction of branching morphogenesis. We will also assay tissue tension at junctions in the bud, and the trunk of developing branches using both YAP overexpression and loss-of-function.
Our laboratory uses a broad spectrum of approaches to ask fundamental questions about how cell and tissues become organized during normal development, and how loss of control of tissue organization and growth results in disease. Our research has allowed us to make significant contributions to understanding the role of Fat cadherins and the Hippo pathway in development and disease. By pairing basic research in Drosophila and mouse with strong collaborations with clinicians, we hope to pave the way for targeted treatments.